for sterile processing
Water Testing
Water quality in the clinical environment plays an important role in infection control and regulatory compliance across the healthcare landscape.
Monitoring and maintaining clinical water helps prevent healthcare-associated infections, meet important standards like ANSI/AAMI ST108, and build trust with patients and staff.
Introducing —Affirm™ Water Tests
BY AGENICS LABS
The Affirm line of water testing solutions brings precision water analysis to healthcare settings, helping facilities meet the routine testing requirements of ANSI/AAMI ST108.
Test kits include all materials needed for sample collection and mailing to the lab. Results provided and stored in complimentary online results portal.
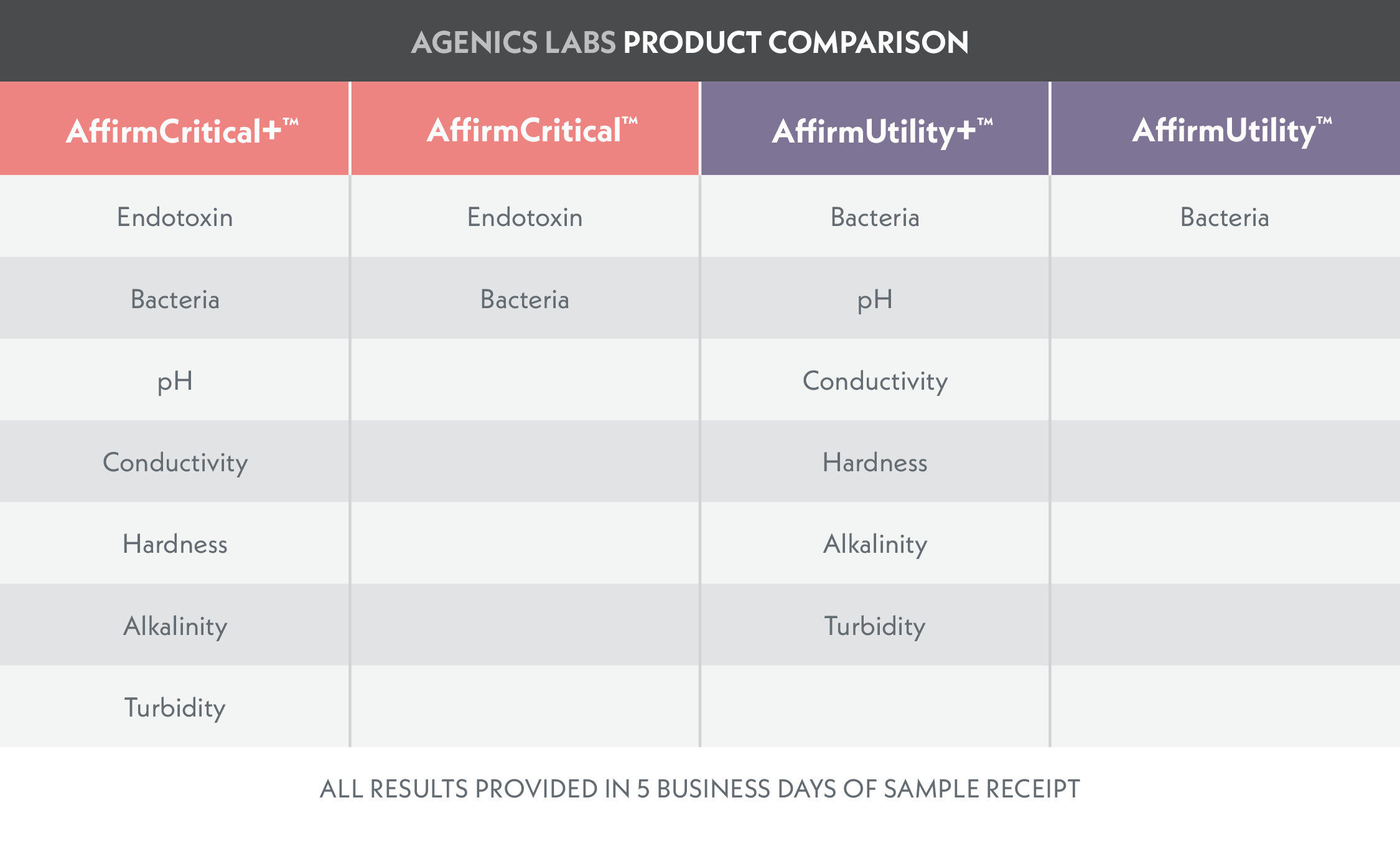
Test kits designed for Critical Water Sources with reporting of endotoxin, bacteria, and other key metrics.
Test kits designed for Critical Water Sources with reporting of bacteria and other key metrics.
Water Testing Made Easy
The Four Steps to Effective Water Management
Assess current water systems and water quality
Implement a water management plan incorporating requirements of the standard
Validate that new systems meet the water quality requirements
Routinely monitor water quality according to the metrics established in the standard
Key Definitions
Critical Water
Critical Water (ST108) is water that meets purity standards for rinsing surgical instruments and medical devices, ensuring patient safety by minimizing organic and inorganic contaminants, microorganisms, and endotoxins.
Utility Water
The initial water coming into a healthcare facility, typically tap water. This water may require additional treatment to meet the quality requirements as outlined in the Standard and can be used for general cleaning tasks such as flushing, washing, and rinsing medical devices.
Water Management Program
A protocol ensuring water quality in medical device processing. An effective program involves a cross-functional team, a risk-based approach, defined roles, verification methods, and documentation standards.
Endotoxins
A toxin present inside a bacterial cell. When a cell dies and disintegrates, the toxin is released and can be responsible for inflammation or some diseases. After bacteria is killed by a water treatment system (such as with UV) the endotoxins must be filtered out. Measured by Endotoxin Unit (EU).
Steam Water
Water that is converted into steam whether from a central steam boiler system or local steam generator for use in medical device sterilization. This water must meet requirements as outlined in the Standard and tested as steam condensate.
Bacteria
A single cell living organism that can cause some diseases. Agenics’ bacteria tests looks specifically for aerobic, heterotrophic bacteria that grows in low nutrient environments, such as water.
Water Quality Standards for Routine Monitoring
For ST108 Compliance
FAQs
-
The Association for the Advancement of Medical Instrumentation (AAMI) introduced ST108, an evolution of AAMI TIR34, to advance water quality in healthcare settings by establishing clear requirements for the water used in medical device processing. The standard explains proper maintenance of water used in the processing of medical devices, including sterilization, cleaning, rinsing, and disinfecting.
-
PATIENTS: Reduce patient risk of infection or toxicity exposure response caused by microbial or endotoxin contamination on devices.
DEVICES: Improve performance and longevity of equipment by reducing device corrosion, pitting, or scaling of equipment
FACILITIES: Improve water system effectiveness while reducing maintenance costs caused by scaling and build-up
-
Considered a best practice for patient safety, compliance with ST108 is required by accrediting organizations including The Joint Commission and DNV.
-
Head to AAMI.org and search AAMI ST108:2023 - Water for Processing of Medical Devices
Resources
Critical Submission Form & IFU
Utility Submission Form & IFU
Affirm Sell Sheet
ST108 Overview
“The importance of monitoring water quality to prevent problems with microbial proliferation cannot be overemphasized.”
— Provided courtesy of AAMI